NMIN Strategic Initiatives Funding
Open, ongoing call for applications
NMIN’s Strategic Initiatives (SI) program supports rapid responses to just-in-time research, commercialization or knowledge mobilization opportunities.
Budgets may be requested to a maximum of $50,000. Projects must be achievable within a one-year timeframe and be aligned with NMIN’s mission and vision.
This is an open competition. Researchers within and outside of the NMIN Network are encouraged to apply.
In addition to following the SI Application Guidelines, applicants are encouraged to consult with the NMIN Research Theme Lead whose program best aligns with the proposed initiative prior to submitting a Proposal Overview.
Proposal Overview submissions will be reviewed by the NMIN Administrative Centre. Only applicants whose Proposal Overviews are most consistent with NMIN’s scientific and strategic priorities will be invited to submit a full SI Application.
Contact the Manager of Research & Partnerships with any questions regarding the SI program.
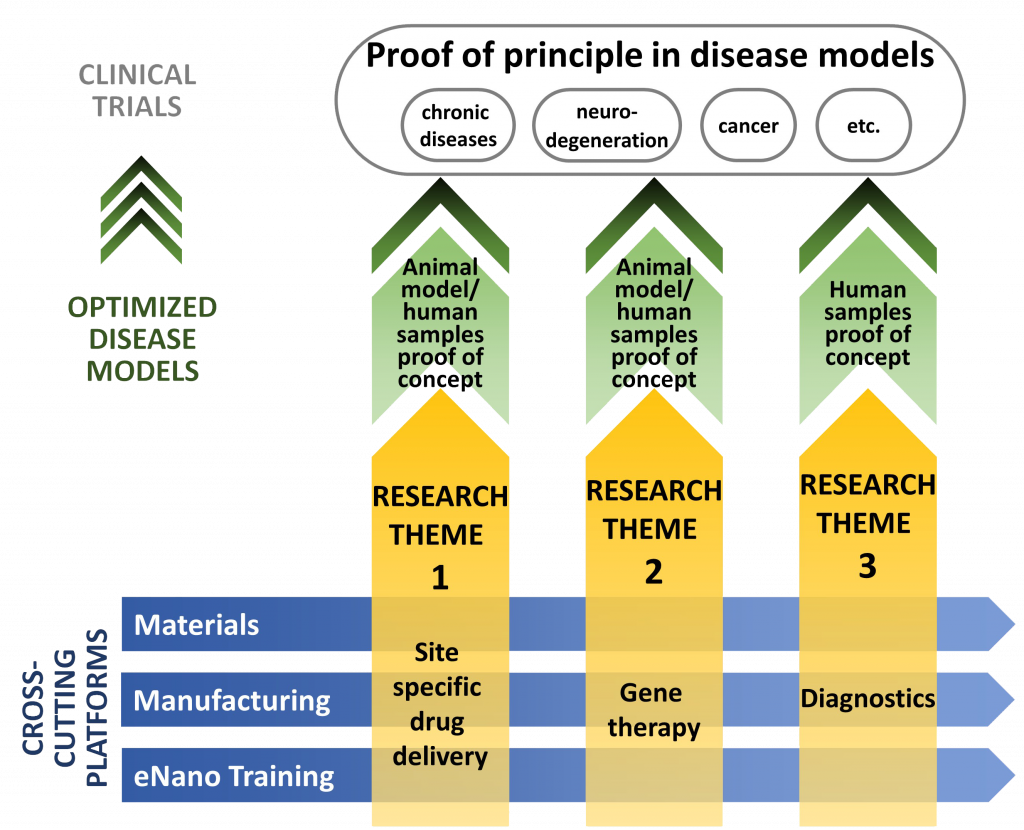
NMIN’s former research program was organized around three themes:
Theme I: Targeted Drug Delivery
Nanomedicines that deliver small molecule drugs more accurately to disease sites, dramatically enhancing the therapeutic properties of small molecule drugs such as anticancer drugs.
Theme II: Gene Therapy
Nanomedicines that enable big molecules, particularly nucleic acid-based drugs, to be used therapeutically, enabling gene therapies to treat most diseases.
Theme III: Diagnostics
Nanotechnologies to detect disease earlier and more accurately, improving preventive healthcare and enabling precision medicine.
In these videos, NMIN Research Leaders introduce the Network’s three thematic research areas
Theme I: Targeted Drug Delivery 101
Theme II: Gene Therapy 101
Theme III: Diagnostics 101
The projects in NMIN’s Research Themes were supported by three Core Facilities:
NanoCore
The Translational NanoMedicines Formulation and Characterization Core Facility (NanoCore) facilitated NMIN’s vision of translating nanomedicines to the clinic by providing state-of-the-art nanoparticle formulations and a standardized nanomedicines characterization service to enable potent therapies that can be readily manufactured. Read more
PharmaCore
The Preclinical, Scale-up Manufacturing & Project Management Core Facility (PharmaCore) supported in vitro and in vivo studies as well as scale-up, stability testing and manufacturing needs to help exemplify commercial potential. PharmaCore helped identify the best potential nanomedicines for commercial development, facilitated collaboration and helped with the formation of companies. Read more
eHTA
The early Health Technology Assessment (eHTA) Core Facility supported the translation of NMIN-funded innovations from bench to bedside. The Facility provided education, coaching, and contract research services on the use of eHTA to inform a broad range of R&D and commercialization-related activities and decisions. Read more
Additional eHTA resources
eHTA Suitability Checklist: developed by NMIN’s eHTA Platform, this checklist will help you assess the potential value of the health technology emerging from your research, to inform your R&D decisions.
The Drug Development Process in Canada & Technology Readiness Levels
A PharmaCore resource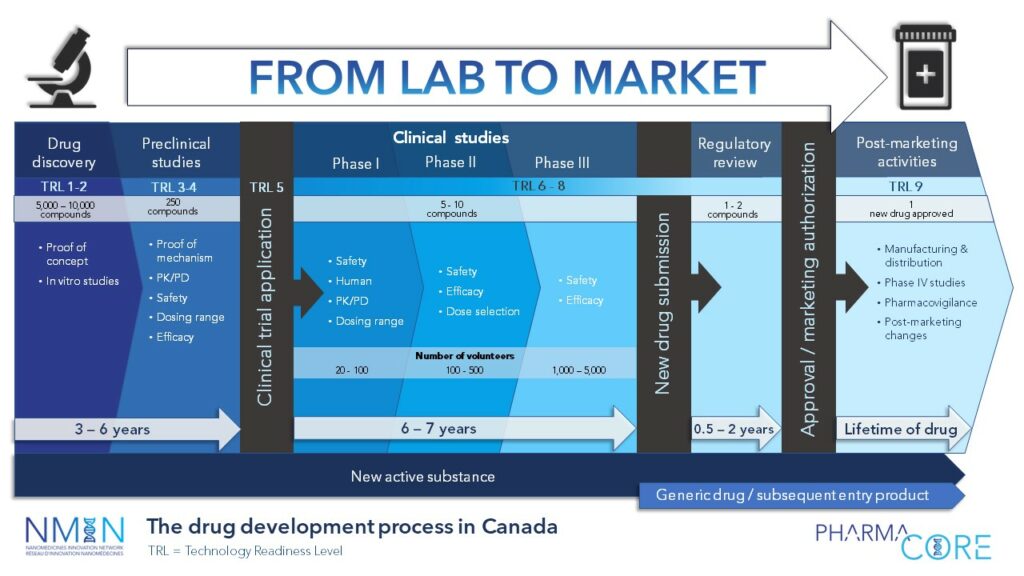
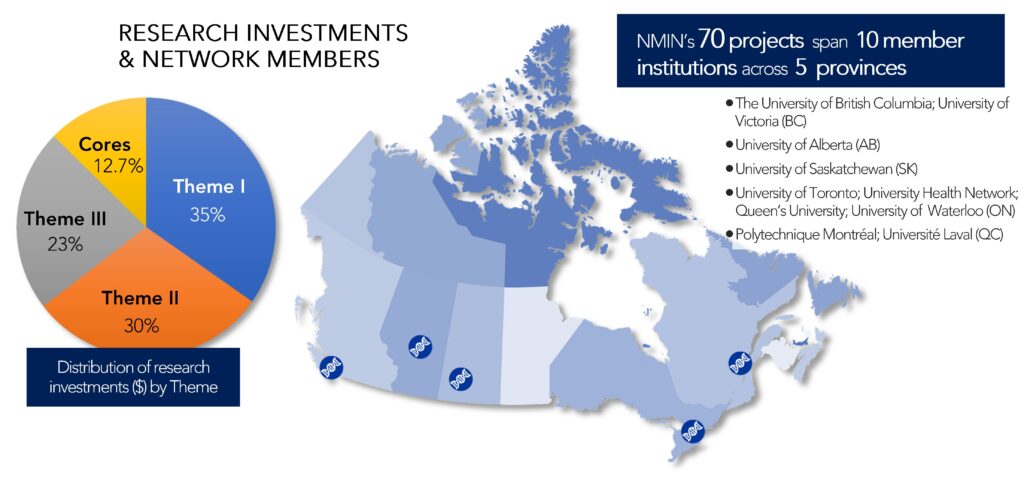
NMIN supported 70 innovative projects, including its three Core Facilities, led by Canadian nanomedicine experts.
These projects and SIs, hosted at 10 research centres across Canada, spanned a range of technologies (from neutrophil encapsulation to LNP systems) applied to a range of illnesses (from cancer to diabetes and eye disease).
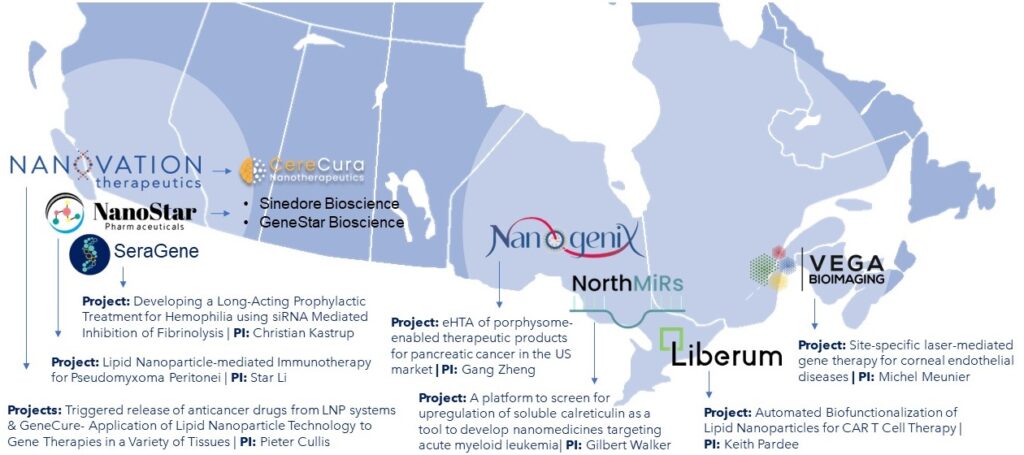
NMIN has spun-off 10 companies to date, emerging from NMIN-funded research projects.
Click on the icons below to view the company’s one-page overview (Fast Facts).
NMIN Strategic Initiatives (SI)
Knowledge and Technology Exchange and Exploitation (KTEE)/Commercialization Support Program
Ongoing call for applications
NMIN’s Strategic Initiatives (SIs) – Knowledge and Technology Exchange and Exploitation (KTEE)/Commercialization Support Program supports Network investigators ready to move forward with commercialization-related and/or knowledge mobilization-related activities.
NMIN’s SIs-KTEE/Commercialization Support Program aims to facilitate: the commercialization of IP (including translation to clinical care); the mobilization of knowledge arising from NMIN-funded research projects; and the growth of Canadian companies (new and existing) in the nanomedicines field with potential to generate social and economic benefits to Canadians.
Budgets may be requested to a maximum of $50,000. Initiatives must be aligned with NMIN’s mission and vision. Proposals with partner funding will be prioritized.
NMIN’s Strategic Initiatives (SIs) – KTEE/Commercialization Support Program aims to:
NMIN’s KTEE/Commercialization support strategy is to encourage NMIN PIs to apply for SI Commercialization funding to secure specialized consulting services with expertise aligned with their project’s specific needs including but not limited to:
To be eligible for NMIN’s Strategic Initiatives (SIs) – KTEE/Commercialization Support Program, you must:









