A new article by NMIN researchers published in the journal Nanoscale sheds light on a potential mechanism by which lipid nanoparticle (LNP) formulations of nucleic acid encapsulate short interfering RNA (siRNA).
“Genetic drugs based on RNA or DNA have a remarkable potential to treat virtually any disease,” comments NMIN Scientific Director Dr. Pieter Cullis, who was lead researcher on the study. “By containing or entrapping therapeutic genetic content, LNPs represent the most advanced delivery systems for such drugs. This research furthers our understanding of how this entrapment occurs within LNPs.”
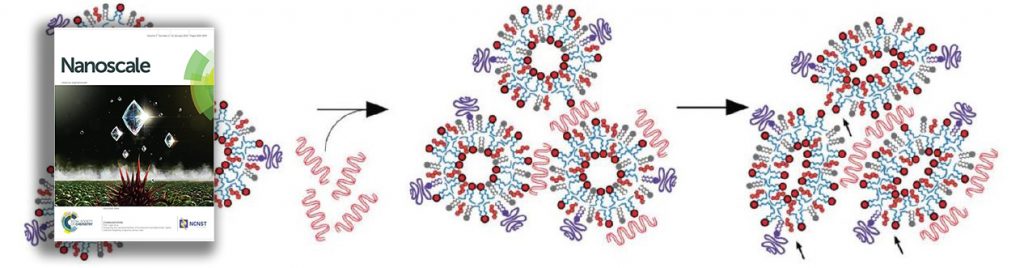
The article provides evidence using cryogenic-transmission electron microscopy and dynamic light scattering to show that siRNA entrapment occurs even without ethanol, the presence of which was previously considered necessary. Their results also suggest that nucleic acid entrapment occurs through inversion of preformed vesicles.
“This finding, in many ways, is an extension of the work in my PhD thesis which showed that LNP adopt vesicular structure at pH 4,” notes Dr. Jayesh Kulkarni, lead author of the study and an NMIN Postdoctoral Fellow.
“The idea of combining preformed vesicles with siRNA on the benchtop is something I had discussed with co-author Dr. Terri Petkau in January 2018 before starting a postdoctoral fellowship with Dr. Blair Leavitt. As we explored the idea it became readily apparent that we had stumbled on a potential mechanism by which entrapment occurs.”
NMIN investigator Dr. Blair R. Leavitt was senior author on the study.
“These findings reconfirm the known therapeutic utility of LNP formulations of nucleic acid, and now extends their utility to ultrasmall-scale formulation screening that reduces the financial burden associated with nucleic acid and protein cargos,” adds Dr. Kulkarni.
“This method is now the principal screening method in the NanoCore and GeneCure. The significance of this NMIN-supported work is substantial.”